Nucleophilic Substitution Reactions of Pyridine
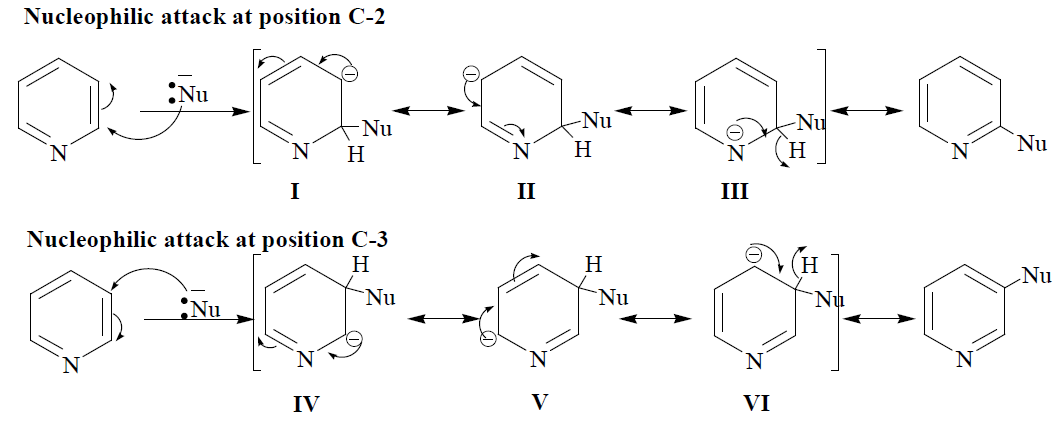
As we have discussed in the previous section that pyridine generally deactivated the aromatic ring towards electrophilic substitution reaction. The deactivation of the aromatic rings towards electrophilic substitution resulted due to the electron-withdrawing nature of the nitrogen atoms. Due to such deactivation, Pyridine also gives nucleophilic substitution reaction. Nucleophilic substitution in pyridine ring occurs at position C-2. Approach of the nucleophilic at position C-2 leads to the formation of three resonating structures(I, II and III); similarly, the approach of nucleophilic at position C-3 also leads to the formation of three resonating structures (IV, V and VI). The resonating structures for intermediate resulting from the attack of the nucleophile at position C-2 are more stable than those of position C-3 since more electronegative nitrogen atoms hold a -ve charge in one of the resonating structures (III) obtained from the attack of the nucleophile at position C-2. Hence, the nucleophilic substitution in pyridine at position C-2 is always favoured. The following mechanism is suggested for the electrophilic attack at position C-2.


Comments
Post a Comment