Electrophilic substitution in Pyridine
Pyridine is also an aromatic compound. It is less aromatic than benzene and pyrrole. Pyridine is usually considered a highly deactivated aromatic nucleus towards electrophilic substitution reactions. Therefore highly vigorous reaction conditions should be used for these reactions to take place. The low reactivity of pyridine towards the electrophilic substitution reactions is due to the following reasons:
- The higher electronegativity of the nitrogen atom reduces electron density on the ring, thus deactivate the ring.
- Pyridine is highly sensitive to acidic medium; it readily forms pyridinium cation with a positive charge on the nitrogen atom. Similarly, electrophile itself may also react with pyridine from the corresponding pyridinium ion. This positive charge on the nitrogen atom decreases the electron density of the nitrogen atom, consequently, the electron density on the ring also decreases.
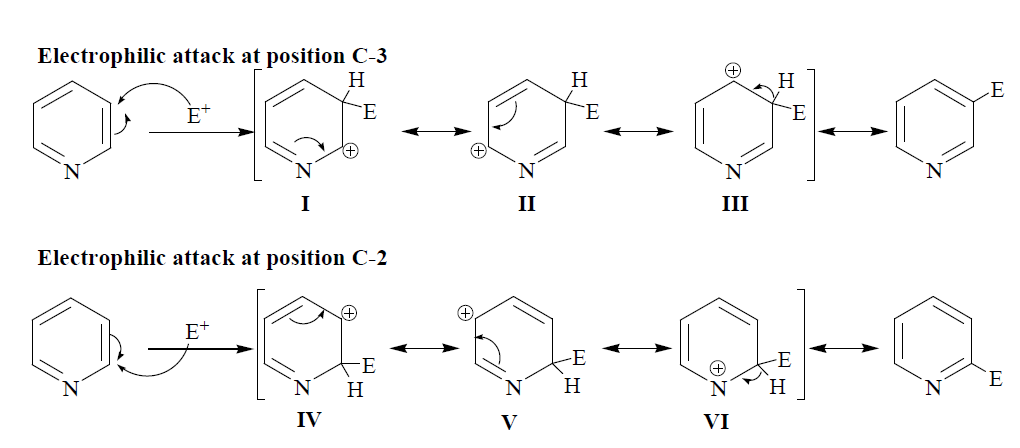
This is the reason that the pyridine undergoes electrophilic substitution at position C-3. Pyridine also gives electrophilic substitution like halogenation, nitration and sulphonation only under drastic conditions. Pyridine does not give Friedel-crafts reaction. The approach of the electrophilic at position c-3 leads to the formation of three resonating structures (I, II and III); similarly, the approach of electrophile at position C-2 also leads to the formation of three resonating structures (IV, V and VI). However, out of the three contributing resonating structures for the intermediate ion resulting from the attack of electrophile at position C-2, structure VI is considered as an unstable resonating form because in resonating structure VI the more electronegative nitrogen atom bears a +ve charge. Because of the unstable nature of one of the resonating structures of the intermediate ion formed during the attack of electrophile at position C-2 than that of the formed during the attack of electrophile at position C-3, the electrophilic substitution in pyridine at position C-3 is always favoured. The following mechanism is suggested for the electrophilic attack at position C-3.
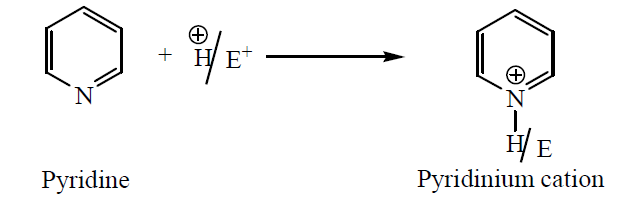
Comments
Post a Comment