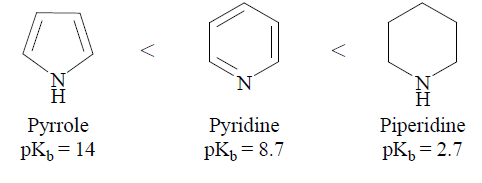
Basicity of Pyridine, Pyrrole
From Experimental studies, it is observed that the pKb values of Pyrrole, Pyridine and Piperidine are ~14, ~8.7 and ~2.7, respectively. Based on the suggested pKb values the priperidine in found as a stronger base than pyridine and pyrrole. Pyrrole is the weakest base among these three heterocyclic bases. The order of basicity of pyrrole, pyridine and piperidine is as given below:
The above order of basicity of pyrrole, pyridine and piperidine can be justified in terms of the structure of these compounds. As we know that the basicity of nitrogen compounds depends upon the availability of lone pair of electrons on the nitrogen atoms. In pyrrole, the lone pair of electron on nitrogen atom exists in the sp2 hybridized orbital of nitrogen and participates in the delocalization, hence does not freely available to cause the basic character of pyrrole. Similar to pyrrole, the lone pair of electrons on the nitrogen atom of pyridine also exists in the sp2 hybridized orbital; however, it does not participate in the delocalization and is available freely to cause the basic character. Although the lone pair of electrons on the nitrogen atom of pyridine is available freely due to the more electronegative character of the sp2 hybridized nitrogen atom (50% s-character) this lone pair is tightly bonded with the nucleus, hence, less available for protonation. However, in piperidine, the lone pair of electrons of nitrogen atom lies in sp3 hybridized orbital of nitrogen. These electrons are less tightly bonded with the nucleus. Therefore, these electrons are readily available for protonation. thus, piperidine is the strongest base among the three.
Comments
Post a Comment